
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
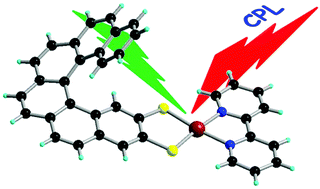
Chiral metal dithiolene complexes represent a family of chiral precursors, which can give rise to molecular materials with properties resulting from the interplay of chirality with conductivity, magnetism, and photophysics. We describe herein the first examples of chiral metal diimine dithiolene complexes, by the use of a platinum(II) centre coordinated by 2,2’-bipyridine and helicene-dithiolene ligands. Straightforward synthesis of racemic and enantiopure complexes allows the preparation of luminescent Pt(bipy) [4] and [6]helicene compounds for which the solid-state structure was determined as well. TD-DFT calculations support the assignment of the low energy bands observed in the UV-vis absorption spectra as mixed metal-ligand-to-ligand charge transfer transitions and confirm that the emission band results from the T1 excited state. Interestingly the enantiopure [6]helicene complexes show CPL activity at room temperature in acetonitrile solutions with anisotropy factors of 3×10-4. | ||||||||
|
||||||||
Introduction of heterocycles in the helical skeleton of helicenes allows modulation of their redox, chiroptical and photophysical properties. Herein, we describe the straightforward preparation and structural characterization by single crystal X-ray diffraction of thiadiazole-[7]helicene, which has been resolved into (M) and (P) enantiomers by chiral HPLC, together with its S-shaped double [4]helicene isomer, as well as the smaller congeners thiadiazole-[5]helicene and benzothiadiazole-anthracene. A copper(II) complex with two thiadiazole-[5]helicene ligands has been structurally characterized and it shows the presence of both (M) and (P) isomers coordinated to the metal centre. The emission properties of the unprecedented heterohelicenes are highly dependent on the helical turn, as the [7]- and [5]helicene are poorly emissive, whereas their isomers, that is, the S-shaped double [4]helicene and thiadiazole-benzanthracene, are luminescent, with quantum efficiencies of 5.4% and 6.5%, respectively. DFT calculations suggest a quenching of the luminescence of enantiopure [7]helicenes through an intersystem crossing mechanism arising from the relaxed excited S1 state. | ||||||||
 |
|
|||||||
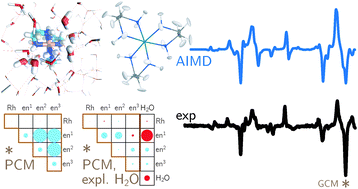
Backscattered Raman optical activity (ROA) spectra are measured for Δ- and Λ-tris-(ethylenediamine)rhodium(III) chloride in aqueous solution. In addition, the spectra of the four possible conformers in the Λ configuration are investigated by ab initio calculations. The Λ(δδδ) conformer is in best agreement with experimental spectra and examined in more details. The two most stable conformers according to the calculations are not compatible with the experimental ROA spectrum. Insights into the origin of observed band intensities are obtained by means of group coupling matrices. The influence of the first solvation shell is explored via an ab initio molecular dynamics simulation. Taking explicit solvent molecules into account further improves the agreement between calculation and experiment. Analysis of selected normal modes using group coupling matrices shows that solvent molecules lead to normal mode rotation and thus contribute to the ROA intensity, whereas the contribution of the Rh can be neglected. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017
